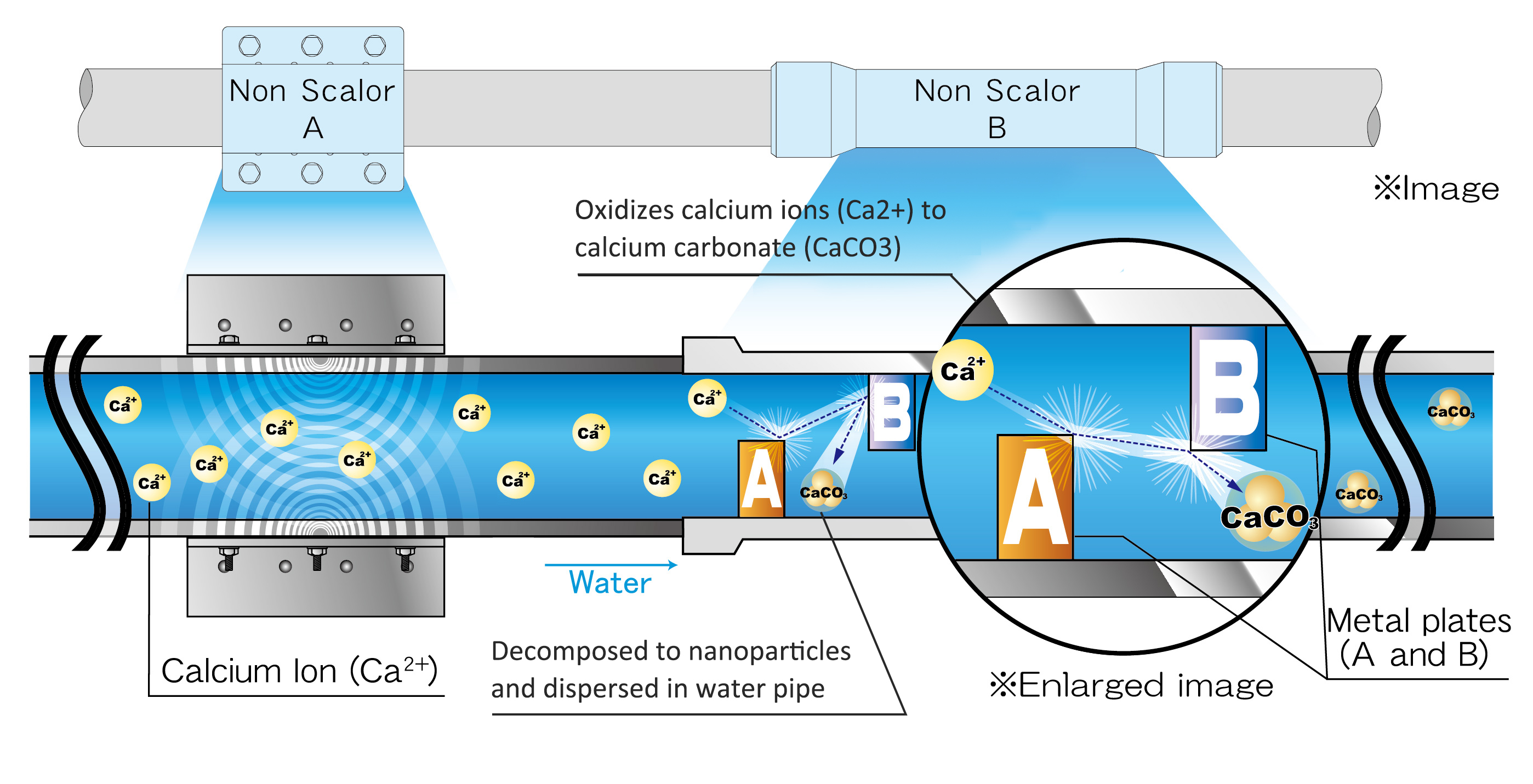
1. Elements of calcium ion are generally dissolved in water as calcium hydroxide {Ca(OH)2)}. When water temperature changes or pH of water turns to acidity, calcium ions bond with free carbonates in water to form a solid calcium carbonate crystal that has water insolubility. The calcium carbonates will eventually adhere as hard calcium scale to inner surface of water pumps, boilers, other heat exchangers and water pipe itself that cause several issues, such as thermal conduction failure.
2. When water passes through a pipe fitted with a Non Scalor unit, elements of calcium ion in water contact with two metallic plates that have different electric polarity inside the unit. The elements are then decomposed to soft nano-particles of calcium carbonate. The nano-particles do not change its state when water temperature and pH are changed, thus calcium scales are prevented from adhering on inner surface of any equipment within water systems.