How does red rust form?
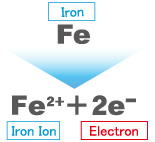
Red rust forms inside iron water pipes when iron is in direct contact with water and oxygen. In other words, red rust forms when iron is deprived of electrons.
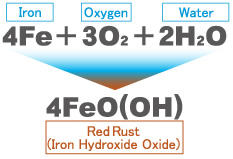
Red rust, FeO(OH), forms when iron reacts with water (H2O) and oxygen (O2).
Red rust easily dissolves in water and causes water discoloration, clogging, corrosion and pipe deterioration, which symptoms lead to water leaks.
How to stop formation of red rust
The other way around, the formation can be stopped if electrons are supplied to iron that lost electrons.
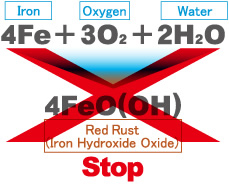
This method is called an electrolytic protection.
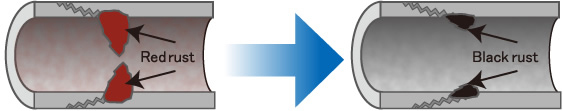
How is red rust reduced to black rust.
Existing red rust can be reduced to black rust by supplying electrons to it continuously.
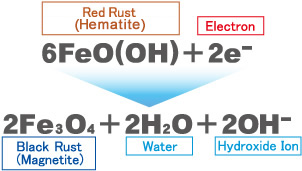
Black rust is passive as a substance: it is highly resistant to oxidation and does not dissolve in water. Its cubic size is 1/10 of that of red rust.
Black rust functions as protective coating on inner surface of pipework. This extends life span of it for more than 40 years.

Disadvantages of conventional pipe cleansings
Conventional pipe cleansing methods, such as flush-out and chemical cleansing, cause red rust to flow out of pipework. They are just putting off the problems. Moreover, a flush-out reduces pipe thickness and wears out inside piping and eventually accelerates further formation of red rust that shortens the life span.
